Sf2 polar or nonpolar lenaka
Explain how a molecule that contains polar bonds can be nonpolar. Answer. As long as the polar bonds are compensated (for example. two identical atoms are found directly across the central atom from one another), the molecule can be nonpolar. PROBLEM \(\PageIndex{2}\)

Polar and Nonpolar Covalent Bonds Characteristics & Differences
To sketch the SF2 Lewis structure by following these instructions: Step-1: SF2 Lewis dot Structure by counting valence electrons on the sulfur atom. Step-2: Lewis Structure of SF2 for counting valence electrons around the terminal fluorine atoms. Step-3: Lewis dot Structure for SF2 generated from step-1 and step-2.

Is SF4 polar or nonpolar Science Education and Tutorials
SF2 is polar in nature because the sulfur (2.58) and fluorine (3.98) atoms in the molecule differ in their electronegativity and the molecule has a bent geometrical shape. Therefore, the dipoles of the S-F bond do not cancel out each other and molecules turn out to be polar and contribute some dipole moment.

Polar and nonpolar dielectrics YouTube
Question: Identify each molecule or ion as polar or nonpolar SeF4, BrI5, SF2, CIO3-. Identify each molecule or ion as polar or nonpolar SeF4, BrI5, SF2, CIO3-. There are 2 steps to solve this one.
Sf2 polar or nonpolar limfath
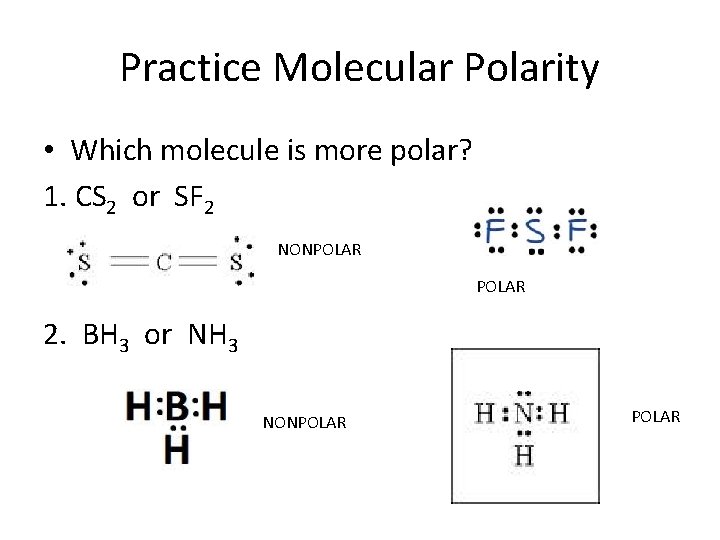
Contents SF2 Valence electrons SF2 Lewis Structure SF2 Hybridization SF2 Molecular Geometry SF2 Shape SF2 Bond Angles Is SF2 polar or nonpolar? SF2 Valence electrons For drawing the Lewis structure for any molecule, we first need to know the total number of valence electrons.

Is SF2 Polar or Nonpolar? (Sulfur Difluoride) Polar, Chemical formula
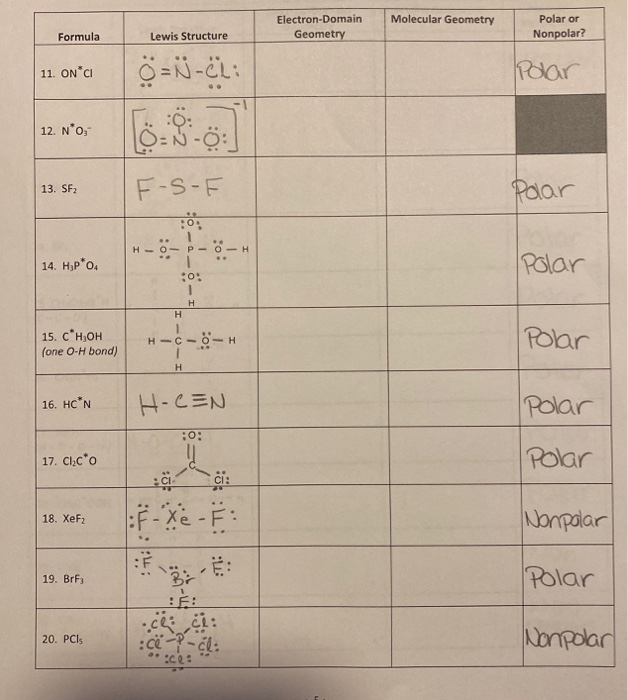
Choose the selection which correctly characterizes all three of the following substances in terms of whether they are polar or nonpolar: CS and Gere and IFS a) CS is polar and Gere is nonpolar and IFs is polar. b) CS is polar and GeHe is nonpolar and IFs is nonpolar. c) CS is polar and Gerais polar and IFs is nonpolar.
Is N2 Polar Or Nonpolar?
S2F2 is polar or non polar? Updated: 8/11/2023 Wiki User ∙ 10y ago Study now See answers (4) Best Answer Copy Think of the sulfite ion as a molecule with its geometry and dipole moment AND a.

SF2 Lewis structure, Molecular geometry, Hybridization, Polar or nonpolar
MakeTheBrainHappy Is SF2 Polar or Nonpolar? Answer: SF2 is a polar molecule due to the presence of lone pair electrons on sulfur which force the molecule to adopt a bent configuration due to electron-electron propulsion.
Sf2 polar or nonpolar jujapress
Hey Guys!In this video, we are going to determine the polarity of Sulphur Difluoride having a chemical formula of SF2. It is made of one Sulphur atom and two.

Is CS2 polar or nonpolar? YouTube
The S-F bond is a highly covalent, polar one. SF2 can be described as a molecule having an elongated geometrical form because of the presence of isolated pairs of the Sulphur atom. The lone pairs pull down Fluorine molecules, which shift the bond angle between 180 and 90 degrees. In the SF2 Lewis structure, the sulfur atom has two bonded.
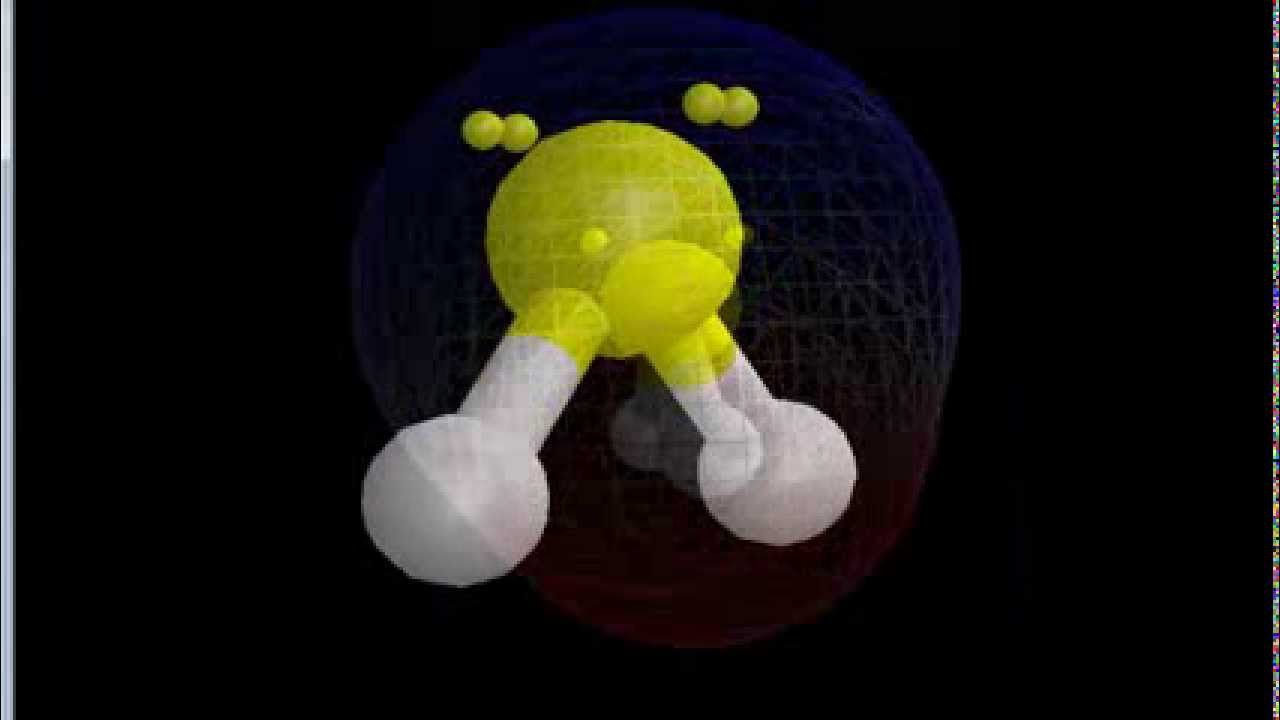
Is SF2 Polar or Nonpolar? YouTube
(Explained in 3 Steps) SF2 is a polar molecule because it has poles of partial positive charge (ẟ+) and partial negative charge (ẟ-) on it. Let me explain this to you in 3 steps! Step #1: Draw the lewis structure Here is a skeleton of SF2 lewis structure and it contains two S-F bonds.
[Solved] image attached 1. Complete the table below. Indicate whether
Step 4: Finally, we need to see if these dipole moments cancel out. In a linear molecule, the dipole moments can cancel out, making the molecule nonpolar. However, in a bent molecule like $\mathrm{SF}_{2}$, the dipole moments do not cancel out. Therefore, the molecule $\mathrm{SF}_{2}$ is \textbf{polar}.

Is SF2 Polar or Nonpolar? Techiescientist
Sulfur difluoride (SF2) is a polar molecule. The central sulfur (S) atom in SF2 is surrounded by two fluorine (F) atoms forming a bent or V-shaped molecule. A fluorine (F) atom is more electronegative than a sulfur (S) atom. Thus both S-F bonds are individually polar in the SF2 molecule and possess a specific dipole moment value.

Sf2 polarity furniturevica
Is SF2 Polar or Nonpolar? Wayne Breslyn 665K subscribers Subscribe 33K views 9 years ago If you look at the Lewis structure for SF2 might appear to be a symmetrical molecule. However, according.
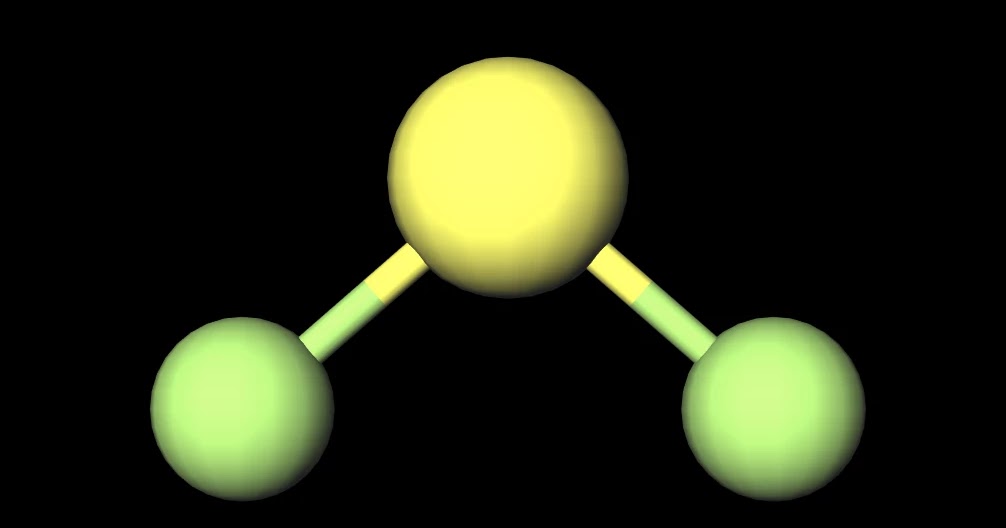
MakeTheBrainHappy Is SF2 Polar or Nonpolar?
Page Contents show How to draw lewis structure of SF2? SF2 Lewis structure is made up of two atoms, sulfur (S), and fluorine (F), the sulfur (S) is in the central position and fluorine (F) atoms are on either side of it. The lewis structure of SF2 contains 16 nonbonding electrons and 4 bonding electrons.
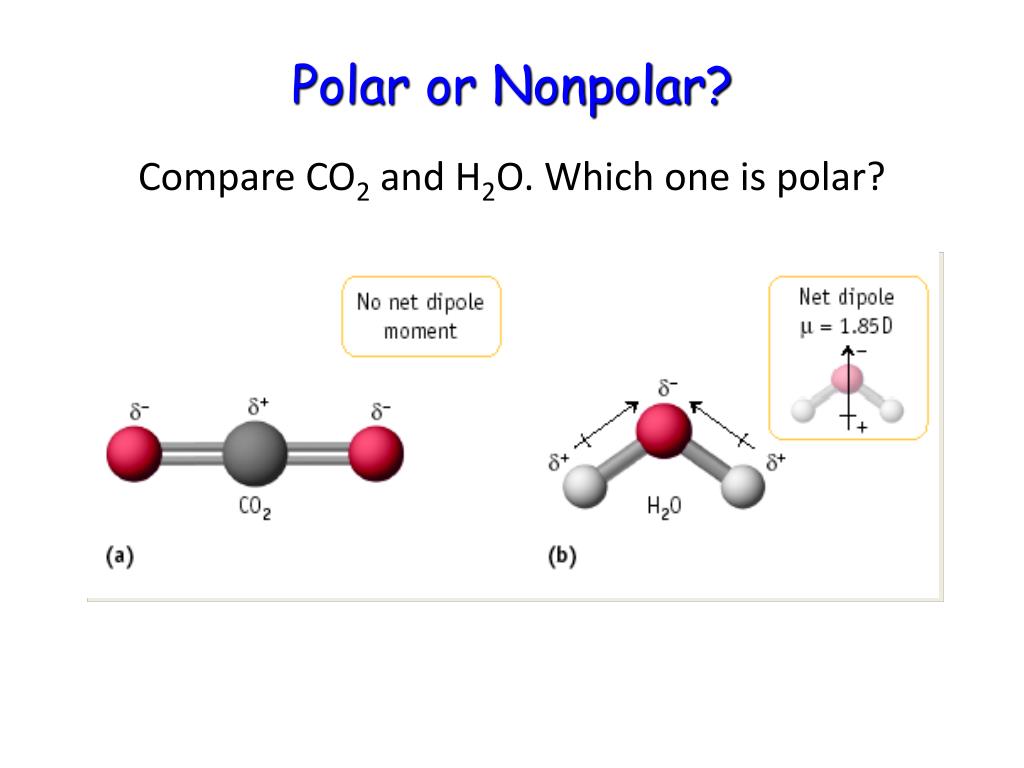
Pcl5 Polar Or Nonpolar Is SF4 consided polar or nonpolar? Quora
SF2 lewis structure is an electron dot representation which can explain many other characteristics related to it. Discover a step by step method to generate SF2 lewis structure is of significance. How to draw the lewis structure for SF2? Count the number of valence electrons